Unharnessed Energy: Invisible Light from the Sky
Our Energetic Atmosphere
by Paul Huber
When you walk outside at night, even in winter, you’re bathed in infrared light from the atmosphere,
averaging a third or more of the radiant energy you’d receive from sunlight. We can’t see it because our
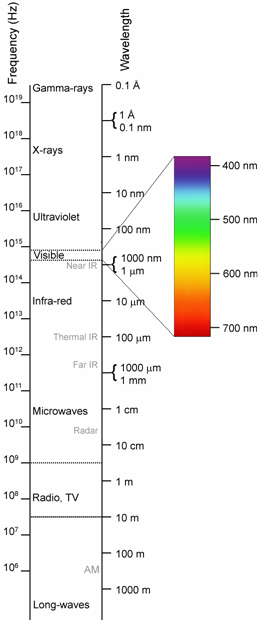
eyes are only sensitive to electromagnetic radiation within the range of about 400 to 700 nanometers (nm)
in wavelength. This light is infrared, in the range of roughly 8 to 12 thousand nanometers, and peaking
around 10,000nm. But do we experience it? If we could turn it off with a switch, I imagine we’d notice a
difference since we’re accustomed to it always being there. However, we don’t feel it like shorter-wave
infrared from a campfire because it’s radiating from an air mass that’s typically colder than our body
temperature. Nonetheless, it’s real energy with a potential for harvesting if we could figure out the
technology.
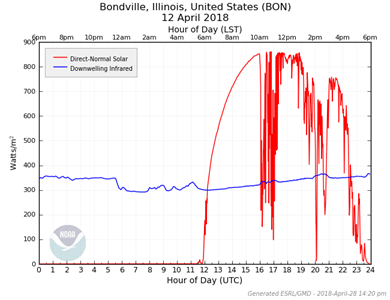
This 24-hour radiation plot shows the energy (in watts per square meter) of direct sunlight and infrared
emitted by the overhead atmosphere. The measurements were recorded by ground based instruments at one of
NOAA’s G-Rad stations. The atmosphere emits infrared because of its temperature above zero Kelvin
(see
thermal radiation).
On this particular day, the near-ground air temperature averaged ~17°C, or ~290K.
Currently, the capacity factor for solar photovoltaic energy is about 27%. That means that over a
typical 24-hour period you will average ~27% of the rated output of a photovoltaic panel – mostly due to
rotation of the Earth, and cloud obstruction to a lesser degree. You only get full output when the
incident angle of sunlight is direct enough and there’s enough transmission through the atmosphere. But
an imaginary collector, designed to convert the much longer wavelength of infrared emitted by the
atmosphere, would have a capacity factor close to 100%. That’s because the emitting air mass is always
there and the infrared is not obstructed by clouds. In fact, if you examine a radiation plot of this
energy you’ll see that heavy cloud cover actually boosts the emission slightly because the clouds
themselves are good emitters.
So why can’t we harness this electromagnetic energy like we do with sunlight? The short answer
is photon energy…
Albert Einstein is known for his theories of relativity (Special & General) but he won his Nobel Prize
(in 1921) for a paper published on the photoelectric effect - "On a heuristic viewpoint concerning the
production and transformation of light" (1905). The research paper built on previous work from scientists,
such as Philipp Lenard and Max Planck, and basically stated that light energy comes in ‘discrete packets’,
later to be called photons in 1926. These photons each have a specific energy value according to their
wavelength, or color when within the visible spectrum. For example, a photon of blue light at 430
nanometers has an energy of 2.88 electron-volts (eV) and a photon of red light at 660nm has an energy of
1.88eV. These photon energies do not change with the intensity of the light – red light from a 10 watt
source would have the same photon energy as from a 1,000 watt source.
This matters because photovoltaic cells operate on a similar principle to the photoelectric effect,
in that electrons are knocked loose within the material by the energy of the photons striking it.
Different materials have different threshold levels that must be met or exceeded for the electrons to be
knocked loose. For example, potassium has a threshold level of ~2.0eV, so photons that strike that
material cannot knock the electrons loose if the photon energy is less than 2.0eV but can if the photon
energy is greater. The amount or intensity of light does not matter.
Current photovoltaic technology began with a discovery published in 1877 - “The Action of Light on
Selenium” in Proceedings of the Royal Society of London, by Adams, W. G. and R. E. Day. While
experimenting with selenium, the researchers accidentally discovered “that light had caused a flow of
electricity through a solid material". Over the following decades the discovery was explored further and
refinements were made, leading to the silicon cells that are most common today in converting sunlight to
electricity. The response of these cells drops off at around 1,100nm because the threshold level of the
silicon is ~1.12eV – photons of lesser energy/longer wavelength cannot free the electrons from their
bonds. In the case of semiconductor materials, such as silicon, this threshold is called the band gap,
and defined as “the energy required to promote a valence electron bound to an atom to become a conduction
electron, which is free to move within the crystal lattice and serve as a charge carrier to conduct
electric current” (Wikipedia). That’s a real challenge if you want to harness infrared energy that peaks
~10,000nm, corresponding to a photon energy ~0.12eV. Nonetheless, it’s real energy, continuously emitted
over 24 hours, that actually averages as much or more than sunlight during that period and isn’t
interrupted by clouds.
Germanium, another semiconductor material, has a lower band gap threshold (~0.66eV) but it’s still
well beyond the photon energy of that infrared light. So in order to capture the energy it’s necessary
to think beyond a photovoltaic effect, as used for sunlight, and consider it in the broader sense as
electromagnetic energy. Within the electromagnetic spectrum, 10 micron (μm) infrared falls between
visible light and microwaves, and beyond microwaves are radio waves that we use for radio and TV
broadcast. The only thing that differentiates these categories is wavelength, which corresponds to
frequency. Radio and microwave energy can be captured with a device called a rectenna
(rectifying antenna), which receives the electromagnetic energy through the antenna and rectifies it
so that it flows in a singular direction as a DC current. This is the basis for a radio receiver and
is easy to do with energy in the megahertz range. Infrared light in the region around 10μm wavelength
has a corresponding frequency in the terahertz range, and this makes it very difficult to harness
technologically, though not impossible. In 2013 a paper was submitted to Nature for publication: “
Design, Optimization and Fabrication of
a 28.3 THz Nano-Rectenna for Infrared Detection and Rectification”. The device described pushes the
current limits of materials and manufacturing, but theoretically could be used for harnessing the infrared
from the atmosphere. There may also be other means that haven’t been considered or discovered yet.
Whether or not it’s necessary or practical to harness this infrared energy that the atmosphere
shines on us is an open question, but it’s a fun thing to think about, both in terms of what’s
available to us from nature and what is possible. □
